Will A Sand Filter Remove Iron From Pool Water
If yous have ever had brownish stains in your swimming pool, you are already all-too-familiar with iron problems. In this article, nosotros discuss how iron gets into our h2o and why it creates stains. Then we will discuss options for managing atomic number 26 to prevent bug like stains and excessive chlorine demand.
Metallic Oxidation creates college chlorine demand
Perhaps the main consequence of having iron and other metals in your water is increased chlorine demand. Metals are the easiest affair for chlorine to oxidize, and therefore the first things to be oxidized. As oxidants, metals like iron reduce chlorine chop-chop. This is why at the beginning of the breakpoint chlorination bend (up to betoken A on the chart below), there is no noticeable increase of chlorine residuals until these "chlorine reducing compounds" like iron are conquered.

Pool operators with fe issues may discover a higher consumption of chlorine, but often it is disregarded. The price may not be noticeably higher considering the pool might be constantly introducing new water with atomic number 26 in it. In other words, information technology's the baseline, and nothing to compare it to. But balance assured, iron absolutely reduces free chlorine in water. Below we will discuss means to control metals like iron, just starting time, let'southward talk well-nigh where fe comes from.
How does iron get into water?
Atomic number 26 is found in about all natural water sources. Co-ordinate to a drinking h2o equipment manufacturer, LennTech, iron is in seawater, rivers, lakes and groundwater also. Information technology up to those of the states who manage and treat water to remove it. So iron usually gets into our swimming pools via the tap h2o. Certain areas of the The states take more iron in their tap water than others. For example, the upper midwestern states (Ohio, Michigan, Indiana, Illinois, Wisconsin and Minnesota) are challenged with notorious iron issues. Swimming pools in those areas must deal with high levels of atomic number 26 out of the faucet, or be plagued with atomic number 26 staining.

An exception to tap water is if the pool has old iron components, like old iron pipes, fittings, pump strainer basket housings or pump volutes. If these components have been worn down (similar having low pH water flowing through them long enough), fe is sure to observe its way into the pool.
There are some exceptions for other metals too, like copper. Copper can get into water from products similar copper algicide, mineral disinfection systems, and from corroding heat exchangers or copper pipes. Pools have turned green from copper before, and sometimes it is mistaken for algae. But permit'south get back to fe.
Dissolved iron in water is mainly present equally ferrous hydroxide (Fe(OH)ii +). Nosotros could attempt to explain the chemical formulas and reactions, only you lot tin can read these three sources if yous are interested in that level of detail. Source 1, Source ii, or you lot can go down the Wikipedia rabbit hole as we did. Just for this article here, we will try and proceed this every bit simple as possible.

Usually, iron gets in our pond pools in a dissolved, soluble state. This means it is mostly invisible and not withal oxidized. If drinking water is chlorinated or chloraminated, yet, some of the iron may become oxidized and have a tinge of brown colour to it. Iron, when oxidized, turns reddish-chocolate-brown, and chlorine is consumed (reduced) in the process. If the iron was not still oxidized in the pipes en route to the pond pool, it certain will be when information technology is met with chlorine or a secondary oxidizer like ozone.
Oxidation is what creates staining.

Oxidation, equally nosotros have discussed in a previous article, is when an oxidizer (like chlorine) steals electrons or protons from an oxidant–similar fe. And so the key to preventing iron staining is to foreclose iron from being oxidized. And to do that, nosotros have a couple of options.
How to prevent atomic number 26 staining
Nosotros can attempt to remove atomic number 26, either from the source water or from the pool itself, and/or we tin can chemically manage it by using a chelating amanuensis or sequest.
Filter Iron out of the tap water
Fe filters be and tin can exist installed on pool make full lines. They do need to be replaced periodically; no filter has unlimited capacity. Such iron filters have a wide range in toll depending on the removal charge per unit you need, and the flow charge per unit of your h2o. For instance, a 2″ fill line on a commercial pool will need a larger, more expensive atomic number 26 filter than a garden hose filling a residential pool.
These filters are a corking choice for initially filling up a puddle (as well chosen a pool startup). The iron–and other metals that may be present in the fill h2o–volition be filtered out prior to adding chlorine to the puddle.
Remove Atomic number 26 from the pool h2o
If your pool is already full and is challenged with iron bug, at that place are options to physically remove atomic number 26 from the pool. Well-nigh solutions in the puddle business are a two-part procedure. The showtime office is to sequester the metals–which basically means to cluster metallic ions together into larger particle sizes–and so apply a filter that can capture the sequestered metals. Such products do be on the market already.
If you accept a D.E. filter, it could be sufficient in and of itself to capture sequestered metals. Removal would be every bit easy as doing a media replacement. This method has difficulty removing already-oxidized fe, notwithstanding. Ferric fe, for case, is non as easy to sequester equally ferrous iron. More than on that in a moment. In order for this plan to work on existing stains, you would demand a style of lifting those stains and getting iron into a state where information technology tin can be more easily sequestered or chelated.
Chelate or Sequester fe to prevent oxidation
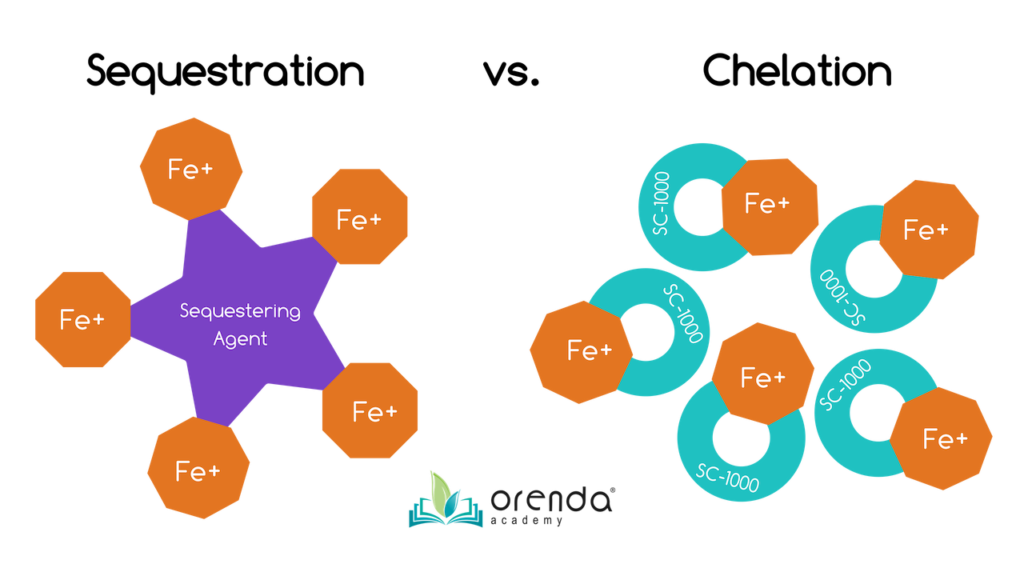
If the problem is non astringent enough that information technology warrants metal removal, you tin still prevent oxidation of those metals. Both sequestering agents and chelating agents can demark to metals and hold onto their protons or electrons. If stains already exist, sequestering and chelation is more difficult because the metals are already oxidized and insoluble. An additional footstep of lifting the stain back into pause is necessary. To practise this, consider using citric acrid (also called ascorbic acrid, or vitamin C).

Our chelating agent NaturallyFREE is proven to chelate ferric and ferrous atomic number 26, simply information technology is very dull to remove stains. It also works better in warmer h2o. If the water is colder than virtually 60ºF, the product will be very deadening or even dormant. Go along this in heed when using NaturallyFREE.
Sequestering and chelation practice not remove metals from the water. They just bind metals to prevent oxidation and staining. You would need some sort of filter to capture and remove the metals. As mentioned before, Regenerative DE filters can capture chelated or sequestered iron. We take even seen information technology happen on sand filters, but only a few times.
Decision
Stains occur when metals go oxidized and become insoluble. Otherwise, metals like iron are in solution or pause, and mostly invisible. Metals are introduced to our pools through the tap water, with few exceptions like copper algicide or corroded metallic equipment in the pump room. The options to solve this problem are either a strategy of removal, or chemically isolating the metals to prevent further oxidation. In either case, if stains are already present, a citric/ascorbic acrid might be needed to lift the stains. Contact us if you need assistance deciding what to exercise.
Will A Sand Filter Remove Iron From Pool Water,
Source: https://naturalpoolproducts.com/iron-swimming-pools/
Posted by: nelsonspermild.blogspot.com


0 Response to "Will A Sand Filter Remove Iron From Pool Water"
Post a Comment